The gold standard
The world of medical device compliance is a tricky one to navigate. At the top level, you have regulations from the FDA in the US, and the MDR/IVDR in Europe. Broken down, you’re then looking at sector-specific requirements like 21 CFR Part 820 in the US, and ISO 13485 + ISO 14972 internationally. Although not always a requirement, ISO 13485 certification is the gold standard, and having this certification allows companies building medical devices to operate in markets across the globe.
The good news is that there are tools out there that can help you along the way. In this blogpost, we’re diving into the world of MedTech, and how to make achieving certification like ISO 13485 and 14972 a much easier, faster, and overall better experience for teams in the industries of digital health and software as a medical device.
We’ll be looking at SoftComply, a QMS platform that seeks to automate regulatory compliance for medical device start-ups by integrating it into Atlassian Confluence and Jira.
We’ll also see how draw.io in Confluence and Jira allows you to create, edit, and customize the process flowcharts and visualizations you need, directly in your QMS space.
If you’re already using Atlassian tools like Confluence and Jira, SoftComply and draw.io are native Marketplace apps that operate seamlessly within your instance, enabling you to be more efficient and focus on the important stuff. They keep all your QMS documentation, templates, and graphic visualizations accessible and secure, as all your data stays directly in your Confluence or Jira instance.
Use SoftComply for all your QMS needs
SoftComply provides several tools for Confluence and Jira to simplify and automate compliance:
- The SoftComply Document Manager is the only Document Management app on Atlassian Confluence Cloud with all the necessary modules. With the Document Manager app you can approve, sign, process, track and manage your electronic documents. The Document Manager has easy-to-use FDA 21 CFR 11-compliant e-signatures for document approvals.
- The SoftComply eQMS has a Quality Manual, a Quality Policy, 25 SOPs and 77 document templates pre-filled to fast-track the development and implementation of your QMS. The SoftComply eQMS is compliant with both the FDA and the MDR regulations.
These tools cover the minimum needs of any company operating in the MedTech sector: a Quality System content and an Electronic Document Management system with electronic signatures.
Your QMS is your single source of truth
ISO 13485 itself is a process-based standard:

Source: ISO 13485:2016(en) Medical devices — Quality management systems — Requirements for regulatory purposes
And when you talk about processes, inputs, outputs and interactions, a graphical visualization is the best way to describe them.
Fortunately, you never have to leave your QMS to create the visualizations that you need: draw.io has you covered. Quickly and intuitively build and store your process flows, diagrams, and any other visualizations you can think of, directly within your single source of truth: Confluence or Jira.
The biggest advantages of creating and storing your diagrams in Confluence and/or Jira are convenience and compliance. Instead of resorting to an external app for your QMS needs, SoftComply keeps everything within your Atlassian tools. This same advantage applies to your draw.io diagrams stored within Confluence/Jira.
Having all your quality documents and diagrams stored within your QMS isn’t just a “nice-to-have” – it’s a must-have from a security, provisioning, and collaborative point of view.
Let’s take a look at some of the specific features below:
Diagramming in Confluence
Control your data and access permissions
You are in control of your data and privacy. The diagrams you create in your browser are saved in Confluence and/or Jira only – not on draw.io servers.
Customize your access levels, depending on your needs. SoftComply provides you with advanced document access control, and whichever documents you provide full, partial, or restricted access to, your draw.io embedded diagrams will follow the exact same settings.
Keep a clear path on your audit trails with revision history
draw.io assists you with your auditing and compliance requirements with its in-editor revision history. This lets you quickly see who edited what and when, and revert your diagram to a previous version if required.
Every change (or reversion) in Confluence and draw.io is captured, effectively providing a timestamp of each version of the page and the embedded diagram. It also ensures that revision history in both Confluence and draw.io are always telling the same story.
Real-time editing in Confluence
draw.io supports real-time collaboration and editing. In the same way that you can collaborate in real-time in Confluence pages, you are able to join your team members in the diagram editor and work together on a particular diagram.
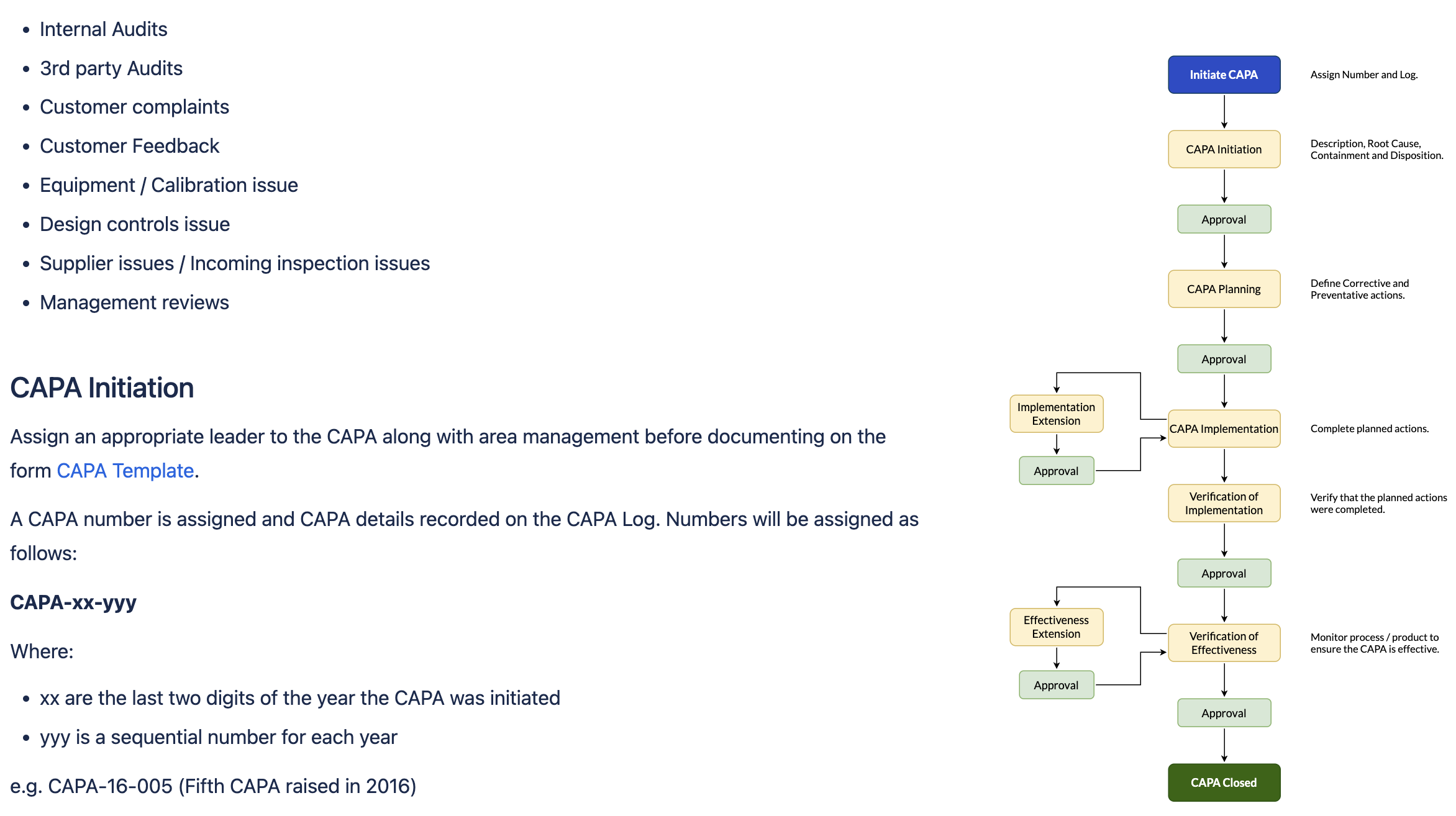
A CAPA SOP. Diagram created in draw.io
Source: SoftComply eQMS
Diagramming in Jira
If you are using both Jira and Confluence, we recommend you try the draw.io app for Confluence, to take advantage of features like revision history, collaborative editing, and our whiteboard editor, which Jira doesn’t support.
If you are using Jira only, you can add and embed diagrams directly in Jira issues. This is ideal for cases where you want to store your visualized processes somewhere central. For example, you might want to store a risk management process (like the one below) in a Jira epic, for anyone working on that project to refer to, to ensure all steps in the process have been adhered to.
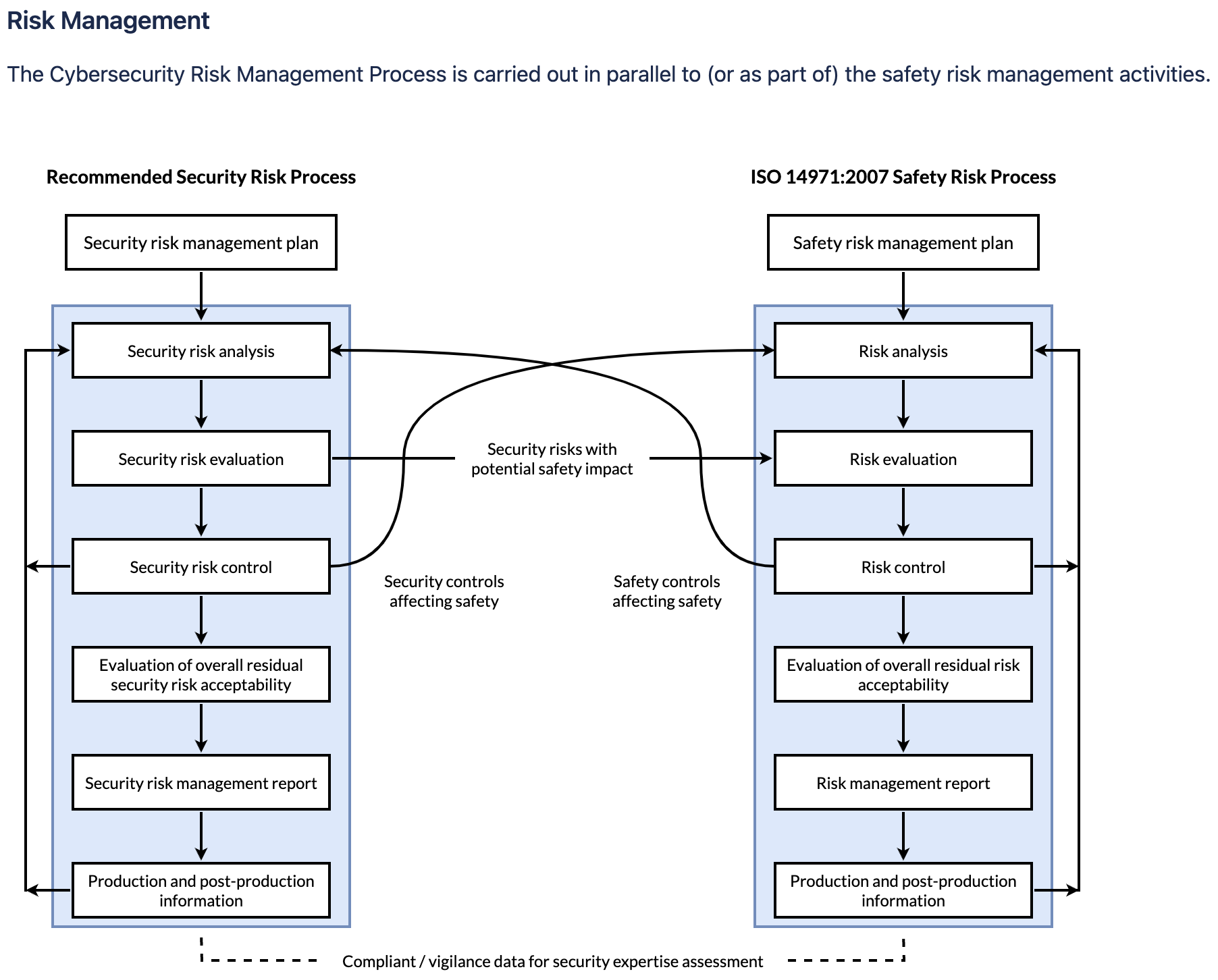
Cybersecurity Risk Management Process. Diagram created in draw.io
Source: AAMI TIR57: 2016 Principles for medical device security—Risk management
Export your diagrams as PDF files
By default, your diagrams exist as living files in your Confluence space. When you have finalized editing a diagram and wish to export this as an offline version, you can quickly convert your diagram to a PDF.
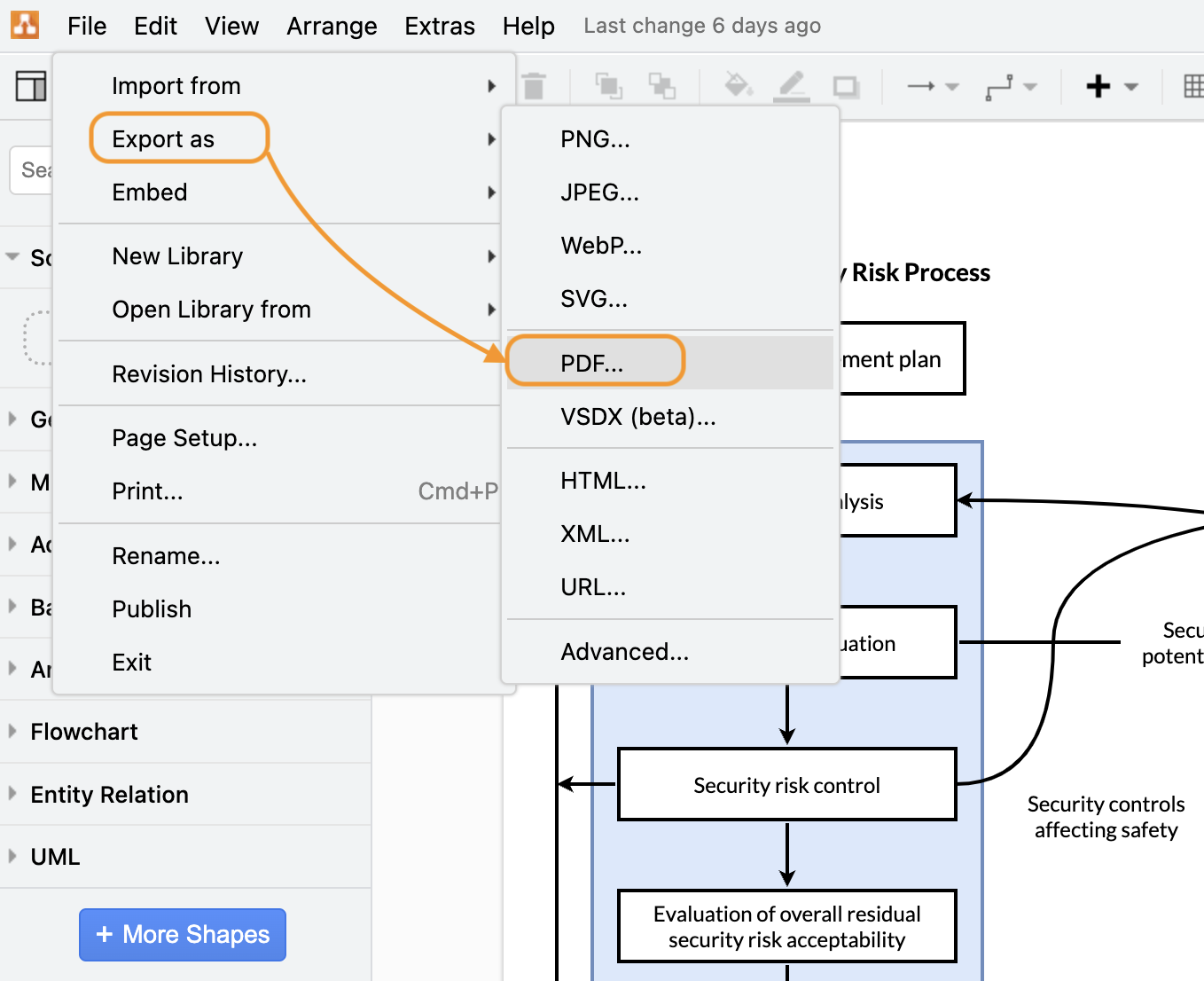
Export your draw.io diagram as a pdf
The best of both worlds
With the SoftComply Document Manager and eQMS, you will have all the required documents and templates to facilitate your QMS implementation, as well as the option to create your own custom forms, all within Atlassian Confluence Cloud.
For visualizations, draw.io is the perfect companion for managing diagrams in Confluence when compliance is required. No external storage and the ability to export to PDF and static versions of the diagrams are crucial to avoiding security and regulatory accidents.
In short, SoftComply and draw.io are two powerful and intuitive tools that will set you on track to achieve your compliance goals.
To find out more, you can get started with a free trial, or book a demo:
You are currently viewing a placeholder content from Youtube. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More InformationWant to dive deeper into the world of draw.io? Access our linktr.ee page to follow us on social media and learn how others use draw.io, as well as pick up some helpful tips and tricks.
Happy diagramming!
Last Updated on December 4, 2023 by Admin